
Fig 1 Difference in layer separation between different centrifugation protocols: (a) 300g for 5 minutes to produce injectable-PRF (i-PRF); (b) 3000g for 8 minutes to produce concentrated-PRF (C-PRF).
PRF, L-PRF, A-PRF, i-PRF, H-PRF, C-PRF, i-PRF, solid-PRF, liquid-PRF. There are a LOT of different types and formulations of PRF—and even more names for them—and that doesn’t even include PRP and other platelet concentrates. Consider the different protocols, tubes, and centrifugation times specific to each one, and it’s no wonder some people get lost in the quagmire (Fig 1). But PRF is too much of a game-changer to ignore it or say it’s too confusing. PRF can be used to promote tissue regeneration and speed healing in cases of recession coverage, periodontal regeneration, bone regeneration, implant dentistry, sinus grafting, oral and maxillofacial surgery, regenerative endodontics, facial esthetics, and multiple applications in medicine. It can literally save limbs in the case of diabetic ulcers (Fig 2), so it’s a no-brainer for adoption into clinical practice.
That being said, there is a lot of information out there, and it can get confusing. This article outlines five of the many different formulations of PRF and their specific protocols. For more information, see Dr Rick Miron’s new book, Understanding Platelet-Rich Fibrin. It covers everything you need to know—and then some.

Fig 2 Chronic and nonhealing foot ulcer (2-year evolution) in a 76-year-old woman with type 2 diabetes with renal failure before (a) and after (b) surgical closure with PRF membranes and regular injection of C-PRF every 10 days for 2.5 months. After seven treatments, the patient remained without ulcer thereafter.
Solid-PRF Membranes and Plugs
 The solid-PRF membrane or plug is created using the horizontal centrifuge and red-cap tubes. The spin time is 700g for 8 minutes, after which a clot is formed. Immediately after centrifugation, the tube lid can be removed to favor clotting via oxygenation. After 5 minutes, the membrane is removed from the tube and the red layer is gently peeled or cut off with scissors, being careful not to cut the buffy coat zone rich in cells (Fig 3). The clot is then transferred to a PRF box, and compression is applied with the PRF box lid (if plugs are desired, cylindric inserts are used to add compression until the piston is flush with the outer rim; Fig 4). Within 2 minutes, the PRF membranes (or plugs) are dehydrated and ready for use. Watch this video to see how it’s done.
The solid-PRF membrane or plug is created using the horizontal centrifuge and red-cap tubes. The spin time is 700g for 8 minutes, after which a clot is formed. Immediately after centrifugation, the tube lid can be removed to favor clotting via oxygenation. After 5 minutes, the membrane is removed from the tube and the red layer is gently peeled or cut off with scissors, being careful not to cut the buffy coat zone rich in cells (Fig 3). The clot is then transferred to a PRF box, and compression is applied with the PRF box lid (if plugs are desired, cylindric inserts are used to add compression until the piston is flush with the outer rim; Fig 4). Within 2 minutes, the PRF membranes (or plugs) are dehydrated and ready for use. Watch this video to see how it’s done.

Fig 3 When removing the clot and trimming the red corpuscle layer, be careful not to cut the platelet-rich buffy coat zone!

Fig 4 PRF membranes being placed into cylindric inserts and compressed into plugs.
Applications for solid-PRF include periodontal surgery, sinus grafting, ridge augmentation, and other surgical wound closures.
Liquid-PRF
 Liquid-PRF is created using a horizontal centrifuge and white- or blue-cap tubes. The spin time is 300g for 5 minutes. The lids should NOT be removed in this case, because oxygenation will speed clotting. An 18G, 1.5-inch syringe needle is used to penetrate the lid of the tube and collect the liquid-PRF. It is important to draw as near to the buffy coat zone as possible and even slightly within this layer (Fig 5). Liquid i-PRF is then ready for injection purposes.
Liquid-PRF is created using a horizontal centrifuge and white- or blue-cap tubes. The spin time is 300g for 5 minutes. The lids should NOT be removed in this case, because oxygenation will speed clotting. An 18G, 1.5-inch syringe needle is used to penetrate the lid of the tube and collect the liquid-PRF. It is important to draw as near to the buffy coat zone as possible and even slightly within this layer (Fig 5). Liquid i-PRF is then ready for injection purposes.

Fig 5 (a) Liquid-PRF is found in the upper 1- to 2-mL plasma zone. The cell-rich zone is found at the buffy coat. (b) An 18G needle is used to draw up the liquid-PRF into a syringe ready for injection.
Liquid-PRF can be used in a host of surgeries as an adjunctive therapy or to create sticky bone (see next section).
Sticky Bone
 Sticky bone is the term commonly given to the bone graft material with sticky/gummy consistency following the addition of PRF. Two PRF membranes are produced following the solid-PRF protocol, and two tubes of liquid-PRF are also produced following that protocol. The clots are removed from their tubes and cut into small (1-mm) fragments before being mixed with the bone graft material (Fig 6). Only then is the liquid-PRF introduced to the complex to hydrate the entire graft. The sticky bone is then ready for use in bone grafting.
Sticky bone is the term commonly given to the bone graft material with sticky/gummy consistency following the addition of PRF. Two PRF membranes are produced following the solid-PRF protocol, and two tubes of liquid-PRF are also produced following that protocol. The clots are removed from their tubes and cut into small (1-mm) fragments before being mixed with the bone graft material (Fig 6). Only then is the liquid-PRF introduced to the complex to hydrate the entire graft. The sticky bone is then ready for use in bone grafting.

Fig 6 Sticky bone. (a and b) PRF membranes are cut into 1-mm-size pieces and mixed with bone graft material. (c and d) Liquid-PRF is added to hydrate the graft and make it malleable.
C-PRF
 The C-PRF protocol is very similar to the liquid-PRF protocol but it leads to a roughly 10-fold increase in cells and growth factors when compared to whole blood. The spin time is much faster (2000g for 8 minutes), and only the 0.5- to 1-mL layer just above the buffy coat is collected (after discarding the top 3 to 4 mL; Fig 7).
The C-PRF protocol is very similar to the liquid-PRF protocol but it leads to a roughly 10-fold increase in cells and growth factors when compared to whole blood. The spin time is much faster (2000g for 8 minutes), and only the 0.5- to 1-mL layer just above the buffy coat is collected (after discarding the top 3 to 4 mL; Fig 7).

Fig 7 (a and b) Collection of C-PRF at the buffy coat zone.
Bio-Filler
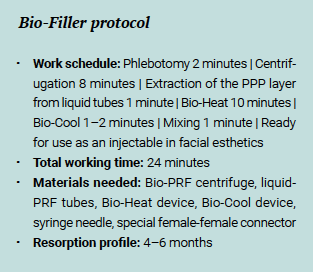 Bio-Filler is fabricated using liquid-PRF tubes spun at 2200g for 8 minutes followed by a Bio-Heat process. From each liquid-PRF tube, the upper 2-mL layer of platelet-poor plasma (PPP) is drawn into a 3-mL syringe and placed in the Bio-Heat device (Bio-PRF) for 10 minutes. After 10 minutes at 75°C, the PPP syringes are placed in the Bio-Cool device (Bio-PRF) for 1 to 2 minutes, while the remaining C-PRF layer in the original tubes is extracted into one syringe per tube. When the albumin gel has cooled down sufficiently, it is mixed back together with the C-PRF layer using 3-mL syringes connected together via a specialized connector. The result is a gel-like texture with extended resorption properties, or e-PRF (Fig 8), which can be used for facial rejuvenation and other facial esthetics procedures.
Bio-Filler is fabricated using liquid-PRF tubes spun at 2200g for 8 minutes followed by a Bio-Heat process. From each liquid-PRF tube, the upper 2-mL layer of platelet-poor plasma (PPP) is drawn into a 3-mL syringe and placed in the Bio-Heat device (Bio-PRF) for 10 minutes. After 10 minutes at 75°C, the PPP syringes are placed in the Bio-Cool device (Bio-PRF) for 1 to 2 minutes, while the remaining C-PRF layer in the original tubes is extracted into one syringe per tube. When the albumin gel has cooled down sufficiently, it is mixed back together with the C-PRF layer using 3-mL syringes connected together via a specialized connector. The result is a gel-like texture with extended resorption properties, or e-PRF (Fig 8), which can be used for facial rejuvenation and other facial esthetics procedures.

Fig 8 Bio-Filler created by mixing albumin gel (a) with C-PRF (b) for facial esthetics purposes (c).
What Now?
These five protocols cover only a handful of the many formulations and possibilities with PRF. It’s up to you to learn more and determine which formulations are valuable for your clinical practice. One thing’s for certain: More applications are on the horizon, and PRF will eventually become part of every clinician’s armamentarium. Are you ready to give it a try?
 Understanding Platelet-Rich Fibrin
Understanding Platelet-Rich Fibrin
Richard J. Miron
Platelet concentrates have been used in medicine for over 20 years now, but the last 5 years have witnessed an explosion in research on platelet-rich fibrin (PRF) because of its ability to promote healing of both bone and soft tissue. This has led to a marked increase in our understanding of PRF therapy with respect to selection of appropriate centrifugation devices, impact of tube chemistry on clotting, the optimization of protocols to better concentrate PRF, and even the ability to extend the working properties of PRF from 2–3 weeks to 4–6 months using a simple heating process. Bringing together expert researchers and clinicians from various dental specialties, this book first explores the biology of PRF and then demonstrates its myriad clinical applications in periodontology, implant dentistry, oral and maxillofacial surgery, endodontics, facial esthetics, and medicine. The true value of this book lies in the blend of data and clinical application, so readers can feel confident knowing that the protocols recommended are fully supported by scientific evidence and demonstrated step by step by clinicians already using them in their daily practice. Even better, supplementary videos throughout showcase these procedures for better understanding. As Dr Robert E. Marx writes, “Understanding Platelet-Rich Fibrin is a book for this decade that transcends all specialties of dentistry and many of medicine,” so it’s definitely one you’ll want to read.
384 pp; 600 illus; ©2021; ISBN 978-1-64724-049-3 (B0493); US $184
 Richard J. Miron, DDS, BMSc, MSc, PhD, Dr med dent, is currently the lead educator and researcher at Advanced PRF Education and is Adjunct Visiting Faculty in the Department of Periodontology at the University of Bern, Switzerland, where he completed his PhD studies. He has published over 250 peer-reviewed articles and lectures internationally on many topics relating to growth factors, bone biomaterials, and guided bone regeneration. Widely considered to be one of the top contributors to research in dentistry, Dr Miron was recognized as the top-ranked researcher on PRF therapy in 2020 according to Expertscape independent review. He also recently won the ITI André Schroeder Prize, the IADR Young Investigator of the Year in the field of implant dentistry, as well as the IADR Socransky Research Award in the field of periodontology. Dr Miron has written five textbooks on regenerative dentistry, and he’s just getting started.
Richard J. Miron, DDS, BMSc, MSc, PhD, Dr med dent, is currently the lead educator and researcher at Advanced PRF Education and is Adjunct Visiting Faculty in the Department of Periodontology at the University of Bern, Switzerland, where he completed his PhD studies. He has published over 250 peer-reviewed articles and lectures internationally on many topics relating to growth factors, bone biomaterials, and guided bone regeneration. Widely considered to be one of the top contributors to research in dentistry, Dr Miron was recognized as the top-ranked researcher on PRF therapy in 2020 according to Expertscape independent review. He also recently won the ITI André Schroeder Prize, the IADR Young Investigator of the Year in the field of implant dentistry, as well as the IADR Socransky Research Award in the field of periodontology. Dr Miron has written five textbooks on regenerative dentistry, and he’s just getting started.
