Biomaterials are a hot topic in modern dentistry. Open any textbook on implant or periodontal surgery, and, in addition to the in-depth descriptions of the surgical protocols, you are bound to find the author’s biomaterial recommendations for each procedure. Gather 20 clinicians in a room and you will likely hear 20 different ways to execute a single procedure. Knowing one way to do something is good—knowing multiple ways to do something is better because it increases versatility and enables the clinician to respond to different clinical indications. That’s the reasoning behind Drs Richard J. Miron and Yufeng Zhang’s new textbook Next-Generation Biomaterials for Bone & Periodontal Regeneration, which takes the opposite approach from other textbooks in that, rather than starting with the clinical indication or surgical procedure and working toward the biomaterial recommendations, they start instead with each biomaterial and work outward from there.
“There are so many textbooks that are on implants or bone grafting or soft tissue management,” says Richard J. Miron, DDS, BMSc, MSc, PhD, Dr med dent, “but there hasn’t really been a textbook that was dedicated to bone and periodontal biomaterials and how and when to use them effectively. When it comes to biomaterials, one thing that is difficult for the average clinician to figure out is that every single company selling biomaterials will tell you their biomaterial can be utilized for every clinical procedure. They promote the perfect all-purpose material, but the reality is that it’s likely not the case. And in textbooks and courses, the author or presenter will provide their clinical preference, but never in the great context of all the available options for that procedure.”
What Drs Miron and Zhang have done differently is break down the biologic background of each biomaterial chapter by chapter. The result is a text that provides the detailed information about each unique biomaterial that will best equip the clinician to use that biomaterial most effectively in any clinical situation for which it is indicated. Again, knowing the steps to do something is good, but knowing the reasoning behind each step is better. If you don’t know how a biomaterial works, you won’t be able to alter how you use it in different clinical indications to achieve the best results.
“One thing that happens,” Dr Miron explains, “is that when people go to dental school, they learn how to do something and get comfortable doing each procedure a certain way and continue doing it that way for the remainder of their careers, which is fine. You always want to perform surgery in an effective way with what works well in your hands and based on your educational and clinical experiences. But because the field of dentistry has been progressing so rapidly, especially in recent years, what happens is what we learned 10 years ago becomes outdated, new tools become available, and new biomaterials facilitate and improve our field, yet it remains difficult to learn all this new knowledge effectively. Furthermore, marketing from companies is at an all-time high, often not providing the best available data or advice to the clinician. We have to close this gap somehow, and the best way is to teach experienced clinicians using evidence-based biomaterials and provide the information they need to make their own rational decisions.”
In their book, Drs Miron and Zhang essentially provide all of the information necessary to solve for one equation: For a given procedure, which grafting material, barrier membrane, and growth factor should you consider to achieve optimal results?
“We have literally gone chapter by chapter on each biomaterial,” Dr Miron explains, “and in each chapter we provide background information that delivers the biologic basis for each biomaterial and how it effectively functions and integrates into the human body. By going through the biologic behavior of each biomaterial, the reader is then taught where and why to use it. We’re not saying ‘This is the clinical situation, so which materials should we use?’ We’re saying ‘This is the material, so let’s explain to you how it works, what regenerative properties it has compared to others, and then determine where we should use it.’ Some of these biomaterials have overlapping indications. Because of that, at the very end of the book we got together with all of the clinicians who helped us write the book and developed a chapter that provides biomaterial recommendations for each clinical indication. On top of that, because more and more clinicians are practicing holistic dentistry in a trend that continues to increase, we have also included alternative options using a holistic approach for every procedure. It’s a different approach with a different perspective that will change how you visualize these biomaterials.”
So if a book like this has never been written before, what made it possible for Drs Miron and Zhang? The answer lies in the unique educational and professional backgrounds of each editor.
“One of the advantages we had in writing this book is that Yufeng Zhang and I are both molecular and cell biologists and dentists,” Dr Miron states, “so we approach each biomaterial from the perspective of both a scientist and a clinician. We both do a lot of preclinical testing on biomaterials, so we have access to all of these different materials in various stages of testing. Since we’ve utilized these materials both preclinically and clinically, it was a good opportunity for us to provide real concrete scientific evidence of each biomaterial and to give our recommendations for their uses. I do not believe any research group anywhere in the world has had such an opportunity to test so many different materials, and we felt it was now necessary to translate what we’ve learned over the past 10 years into an easy-to-read textbook. Our goal was not to simply give recommendations. Our goal was that the dentist better understand each biomaterial to allow them to make better clinical choices for their use as opposed to simply relying on sales reps and vendors to provide their educational information.”
While the number of biomaterials currently available may already seem overwhelming to the clinician, and new ones seem to come to market every other day, this is only the tip of the iceberg. Behind every newly available biomaterial are scores of others that are still trickling through the preclinical and preapproval stages of research and development.
“A lot of clinicians don’t know how long it actually takes to make these products available,” Dr Miron explains. “It can take about 5 years to bring a biomaterial to commercial use, and during that time there has to be the right amount of funding for testing and research. And for some of these new growth factors, we have to do phase I, II, and III clinical studies. There are many steps, and there is a lot of FDA involvement to make sure that the products are safe and that they are properly investigated before commercialization. The process takes a lot of time and money. Then the process is repeated in each country because every country has different standards, and a lot of countries want their own data. With growth factors, it’s an even bigger hurdle because these are often considered a kind of ‘drug,’ so it takes a lot more money to bring them to market with FDA clearance. That’s unfortunate because we do have growth factors in development that are stimulating either bone or periodontal regeneration better than the current standards, but a great deal of investment and time is needed to commercialize them.”
Another side effect of the lengthy commercialization process is the domino effect: Even once a biomaterial makes it to market in one country, it will still take time for it to become available in others, meaning there are different sets of biomaterials clinically available in different countries.
“I did my PhD in 2009 in Bern, Switzerland,” Dr Miron states, “where we were doing a lot of testing on these biomaterials. A lot of these materials come out first in Europe because it’s a little easier to get CE approval than FDA, so these materials often start in Europe and go through 2 to 3 years of testing before they get brought to the North American market. I still have a lot of colleagues in Bern, and I visit and collaborate with them frequently enough that I can continue participating in their research activities, giving me the opportunity to learn which biomaterials passing through the European process might be worthwhile to bring through the FDA process. That’s something I’ve been highly interested in doing since I would like to close this gap of time between when our colleagues in Europe receive approval to use some of these new exciting materials compared with when we in North America gain access to them. I find this waiting period that typically lasts 2 to 3 years before they become available in North America to be too lengthy, so I’ve been trying to work more closely with the FDA to shorten this timeframe.”
Another issue is the difference between standards and cultural norms that may prevent a biomaterial from gaining ground in a country altogether, regardless of clinical data and success as well as how long the biomaterial has been in use. “An example of this is rhBMP-2,” Dr Miron explains, “which is available here in the US but not really used or available in European countries. It’s the same case for allografts. We’re big believers here in North America that you can use somebody else’s cadaver bone, whereas in Europe it’s not very frequently used. Clinicians there would rather use bovine or synthetic bone (xenograft). It’s just a difference in philosophy really, and the nice thing about the book is that we’ve summarized things from many perspectives and from many different coauthors. The book has more than 70 different coauthors, and they come from Asia, Europe, North America, South America, and Africa. We have contributions from people all over the world who have a good understanding of certain biomaterials since they are using them more frequently in their respective countries, and their points are discussed equally. We can all learn from what somebody else is doing, and it was great to receive so many perspectives on these biomaterials.”
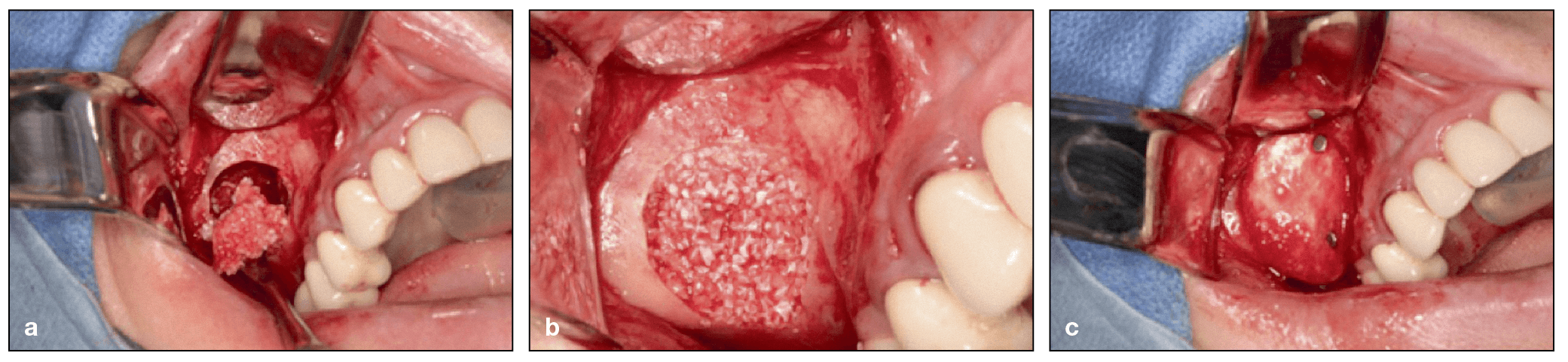
Lateral window sinus augmentation procedure via a delayed approach utilizing a 1:1 mixture of deproteinized bovine bone mineral (DBBM) and freeze-dried bone allograft (FDBA). DBBM (Bio-Oss) and FDBA (MinerOss, BioHorizons) are mixed in a 1:1 ratio and inserted into the sinus. (b) The grafting material is packed into the sinus. (c) A barrier membrane with fixation is used to cover the window. (Case performed by Dr Michael A. Pikos.)
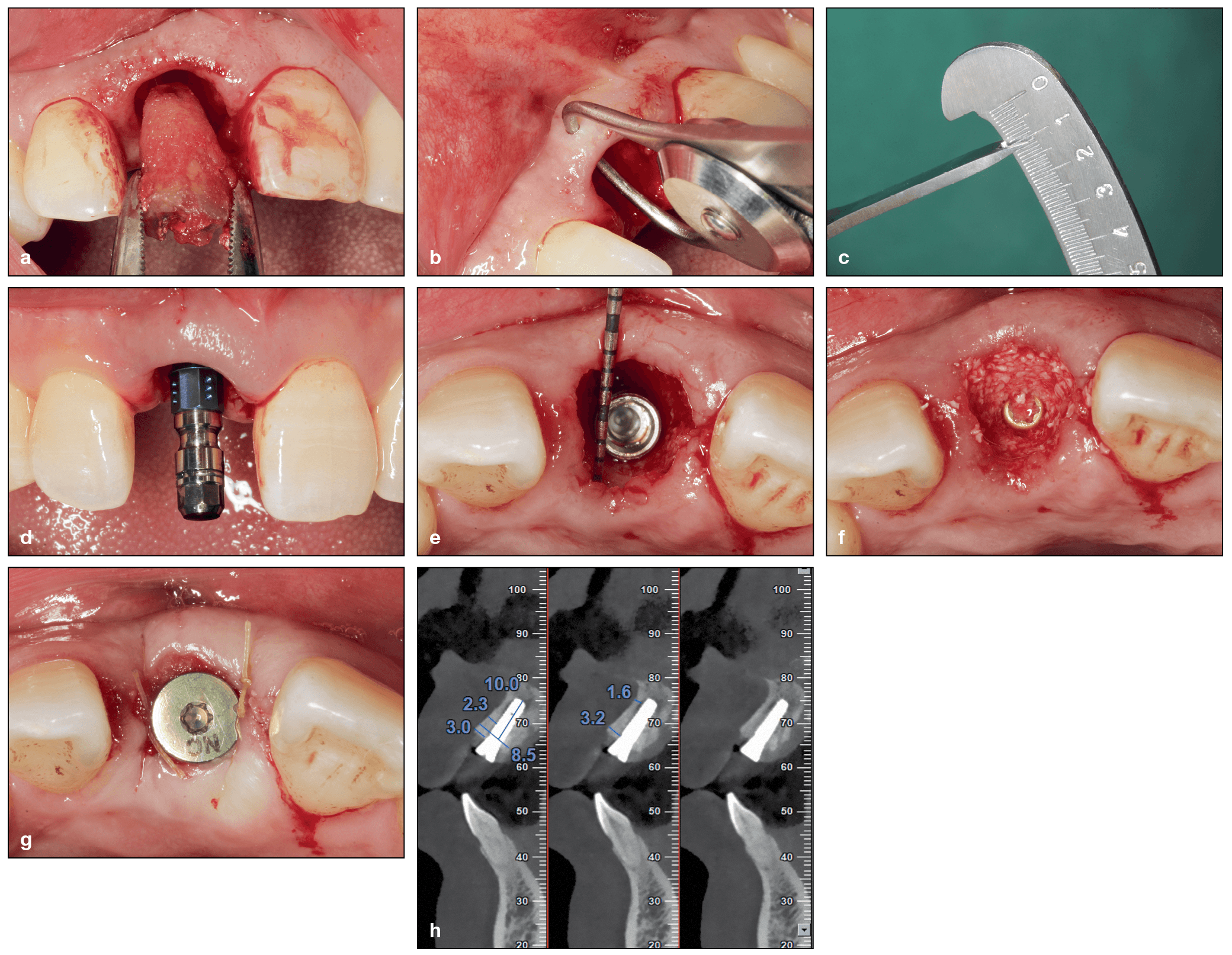
Immediate implant placement in the esthetic zone using a xenograft (DBBM) to pack the gap. (a) Minimally traumatic tooth extraction of a fractured maxillary right central incisor. (b and c) The buccopalatal width of the buccal bone was measured at approximately 1 mm. (d) Implant placement. (e) The implant was placed palatally in the correct 3D position. A buccal gap was created following implant placement. (f) The buccal gap is filled with DBBM. (g) A cover screw is placed with a collagen sponge. (h) Final CBCT after implant placement demonstrating adequate buccal bone. (Case performed by Dr Yufeng Zhang.)
Intended for a global audience and with a watchful eye on the future, the book’s contents are not limited to what is currently and widely commercially available. That’s where the “next-generation” aspect comes in: in addition to gold-standard materials and other available biomaterials, the book also dedicates ample space to new and emerging biomaterials that, while not available everywhere today, are certainly exciting and something clinicians should be aware of for the near future.
Dr Miron provides a few examples. “Today in Europe, they’re using a lot of hyaluronic acid for periodontal regeneration. There are a lot of benefits to that product, and it’s being heavily utilized and studied in Europe, but it’s not being used at all today in the United States. Nobody’s talking about it, nobody’s really doing any experiments with it, and it’s not used even in university settings. In Japan they’re doing great things with atelocollagen right now, which can be processed from the collagen components within the bone structure to create a less-immunogenic bone graft material that favors less of a foreign body reaction. We need to be learning about that here in North America, and most clinicians have not even heard of it! We wanted to collaborate with all of these different colleagues around the world and in such an internationally minded book to allow for more widespread sharing of knowledge.”
Up and Coming: Biomaterials to Watch
With a large mix of biomaterials covered ranging from those still in various stages of development and those clinicians can put to use today, it begs the question: Which of these next-generation biomaterials will become the new standard in coming years?
“The whole concept of this book,” Dr Miron explains, “is that we wanted to develop a textbook that was simultaneously looking at the present and to the future. In this edition of the book, the biomaterials that are just being launched right now and are treated in the text as ‘next-generation biomaterials’ have a lot of preclinical data but not a lot of clinical data. Our plan is to update the book in 5 to 8 years with new biomaterials. By then, there will be a lot more clinical data for many of these biomaterials that are considered new in 2019, and the biomaterials that are considered future today may very well be the standard of care in 5 to 10 years. And by that time, there will certainly be new and exciting developments of next-generation biomaterials, and the cycle will continue with each edition of the book. It should be an exciting project to work on for years to come.”
So taking into account the long R&D gestation period and the long-shot statistics of commercial success for each biomaterial, which next-generation biomaterials from this book would Dr Miron place his bet on becoming the new standard in the years to come?
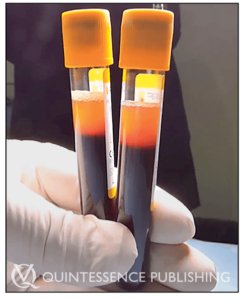
The newer formulation of liquid PRF found in the top 1-mL layer of centrifugation tubes following a 700-rpm spin for 3 minutes. This liquid can be collected in a syringe and reinjected into defect sites or mixed with biomaterials to improve their bioactive properties.
“I think what will grow the fastest and are the most exciting are the more natural biomaterials,” Dr Miron states. “I think that the dentin grinder from chapter 8 of the book—where you actually take the tooth that you just extracted from the patient and grind it up and use it as a dentin particulate graft —is going to grow tremendously over the next 5 to 10 years. I also think that things like platelet concentrates are still under-studied today, and further improvements are necessary going forward. We need to better understand the regenerative potential of each of these biomaterials. One barrier for these natural materials, though, is that they are derived cheaply from the human body. Unfortunately, we’ve learned that the cheaper a biomaterial is, the less commercial involvement they attract. For example, if you extract a tooth and grind it right in the clinic to make a bone graft, your cost is very low. It’s the same with platelet concentrates where you use the patient’s own blood and concentrate growth factors following centrifugation. The problem and downfall with these more ‘natural’ materials is that there isn’t enough money made for companies to support further research and improvement of these biomaterials owing to a general lack of funding. Unfortunately, these materials are more biologic, easier to obtain, and, I would say, probably healthier for you, and they aren’t being studied enough to be viable commercially. And that is a real disadvantage for the field and for patients because they could benefit tremendously from these more natural materials. This is where I think the National Institutes of Health (NIH) should get involved with funding some of these projects, since I do believe it is in the best interest of the national health of our patients.”
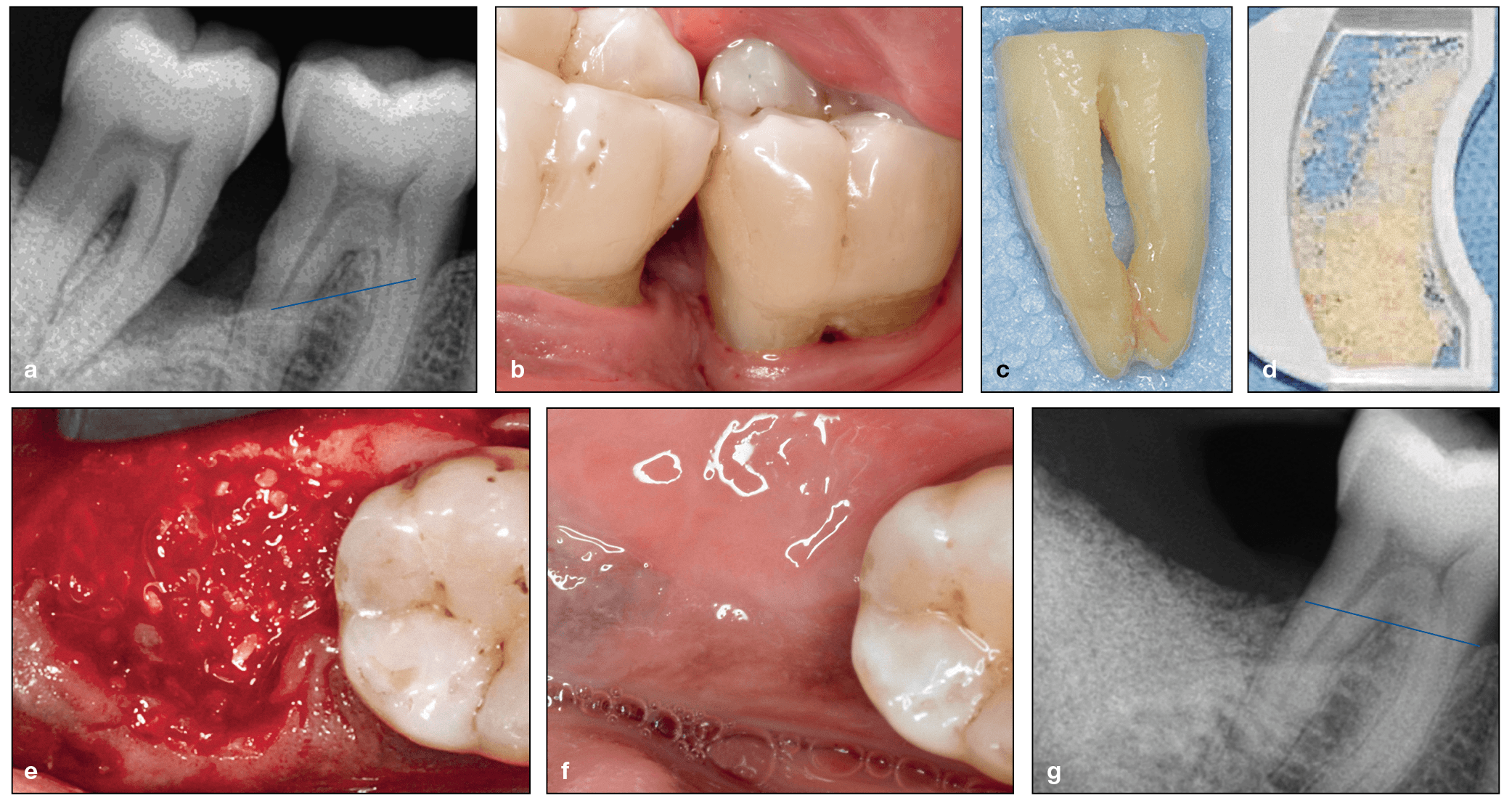
(a) Radiograph demonstrating progressive bone loss of the mandibular left first and second molars, which are periodontally involved. (b) Clinical view of the intrabony defects and exposure of the furcation. (c) The second molar was extracted, and the roots were cleaned. (d) Particulate was prepared using the Smart Dentin Grinder. (e) The particulate dentin was utilized in the extraction socket and grafted against the distal exposed roots of the first molar. Observe the blood that adsorbed onto the dentin particulate graft. (f) Six weeks postextraction, a clinical site next to the first molar shows healthy mucosa. (g) Three months after surgery, the dentin graft is stable within the extraction socket and there is better periodontal support around the first molar. (Case performed by Dr Justin Cifuni.)
Despite the issues these low-profit biomaterials face in gaining wider commercialization, they certainly connect with clinicians.
“The more clinicians become aware of these products, the more they use them, and the more they see a benefit and more interest they create.” Dr Miron explains. “I was in a course taught by Michael Pikos, and he referred to this as ‘going green.’ I laughed so much when I heard him say that because it’s a good way to phrase it: it’s a more organic, more natural way to do things. The advantage to the patient is that you don’t necessarily need to introduce a foreign body material into their body. You don’t have to take a collagen source from the pericardium of a cadaver or the tendon of an animal. You can actually accomplish a lot naturally. I think that this avenue of future research is really exciting, and I think if we can make biomaterials healthier in general for patients, that would be great for our profession. There are certainly indications where you absolutely require a collagen membrane, without question, just like there are indications where you absolutely need an allograft. But whenever we can do things more naturally, it’s less expensive, and if it leads to similar or better outcomes for our patients, then why not?”
While more natural materials provide a marked cost advantage, Dr Miron insists there are other advantages to biomaterials that may cost more, which emphasizes that the greatest benefit lies in having options. “In the book,” he explains, “we also discuss options for biomaterials related to cost. As the field progresses, materials are typically going down in cost. There are still some, especially with regard to growth factors, that are quite expensive, and a perfect example of that is rhBMP-2, which is sold by Medtronic and costs upwards of $600 per case. Now the argument for rhBMP-2 that a lot of expert clinicians such as Robert Marx are making, is that in some indications you can completely replace the need to harvest autogenous bone from the patient’s body by instead using rhBMP-2. You could maybe replace having to get bone from the iliac crest or the tibia and instead use growth factors. You can then present both options to your patient. You’ll notice that in a lot of leading hospitals, and in oral and maxillofacial surgery especially, they’re trying to minimize morbidity of the patient by utilizing some of these newer technologies when it comes to growth factors and recombinant proteins. A lot of patients will go down that route because it makes a lot of sense: pay a little more but avoid a second surgical site with additional morbidity. The good thing is that the more we utilize some of these biomaterials, the lower the costs typically become. I truly believe that the future of medicine in general—not just dentistry—really relates to growth factor use. This is one area that every clinician should be aware of and keeping tabs on.”
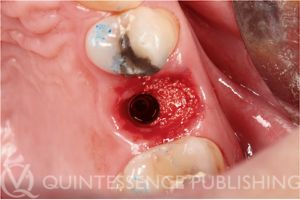
Clinical photograph demonstrating the use of Osopia to fill the buccal gap during immediate implant placement. Because Osopia is both mineralized and osteoinductive, it offers the advantage of inducing rapid new bone formation while resorbing more slowly over time when compared to DFDBA. (Case performed by Dr Albert Barroso Panella.)
But there are plenty more biomaterials in the book worth getting excited about. “Another avenue that I personally find very exciting are new synthetic bone graft materials that are now osteoinductive, meaning the bone graft material is able to form ectopic bone. A simpler explanation is that these materials are kind of super inducers of bone regeneration. Today there are only two FDA-approved osteoinductive biomaterials: a demineralized allograft coming from another human cadaver or rhBMP-2, which is very expensive. Recently, however, a group of researchers in the Netherlands found a way to make osteoinductive synthetic materials. It’s the first time anyone’s ever been able to do that, and we’ve included their research in this book. This material is being used clinically in Europe, and it currently presents itself as being a big breakthrough. It’s already FDA-approved for orthopedic use here in USA, and I’m assuming that it will be approved for dental use very shortly, and it’s going to have a huge impact on the field.”
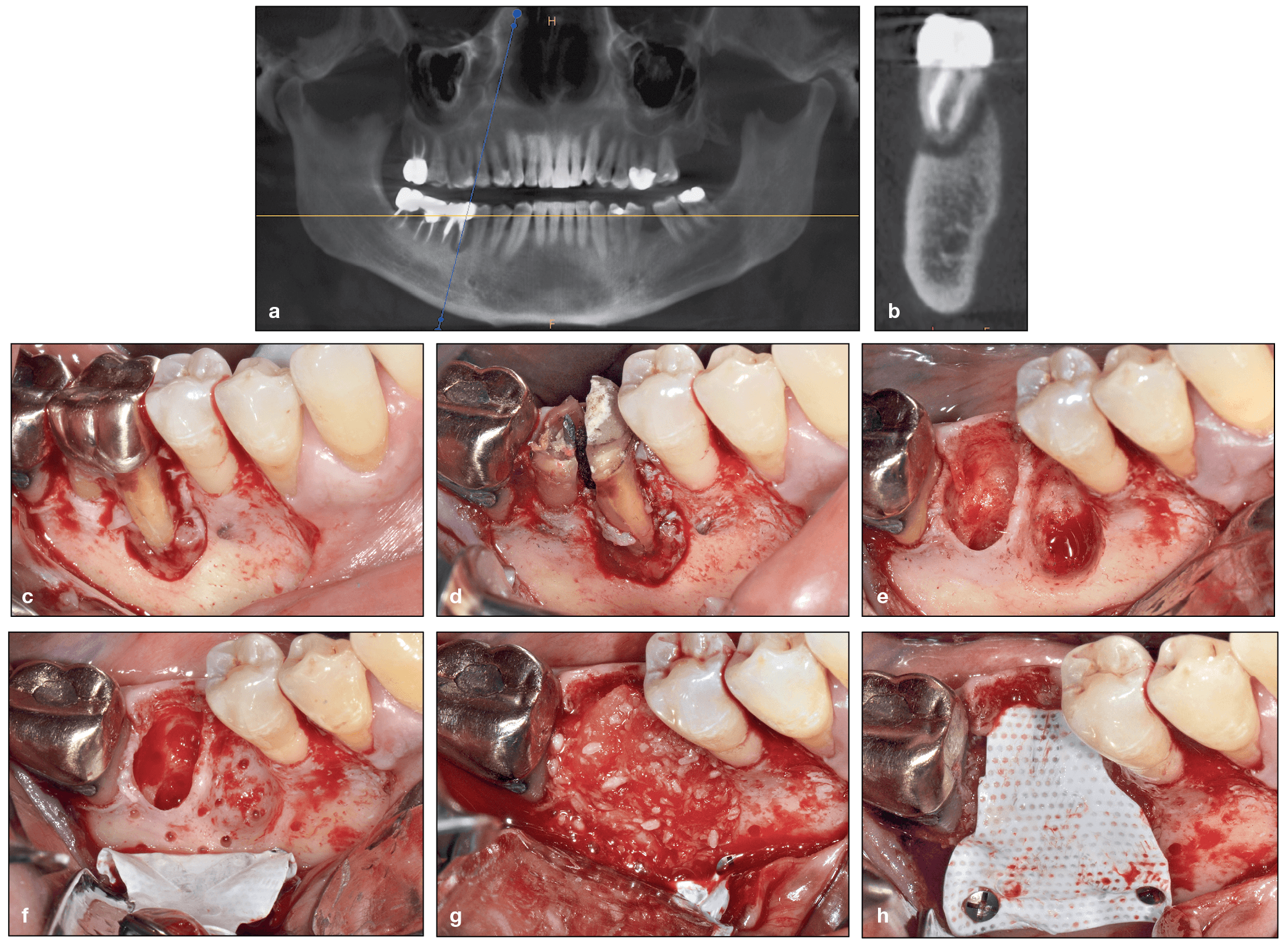
Use of rhBMP-2 following extraction of a mandibular first molar with exposed root surfaces. (a and b) CBCT demonstrating a periapical lesion at the site of the mandibular right first molar and complete loss of the buccal plate. (c) The extent of bone loss is observed after flap elevation. (d and e) Extraction of the mandibular first molar. Note that the tooth was split in half and removed atraumatically. (f) After decortication and intramarrow penetration, a titanium-reinforced dense polytetrafluoroethylene (dPTFE) membrane was fixed apically. (g and h) rhBMP-2 was mixed with an allograft, and the composite graft was used to fill the bone void, after which the membrane was secured.
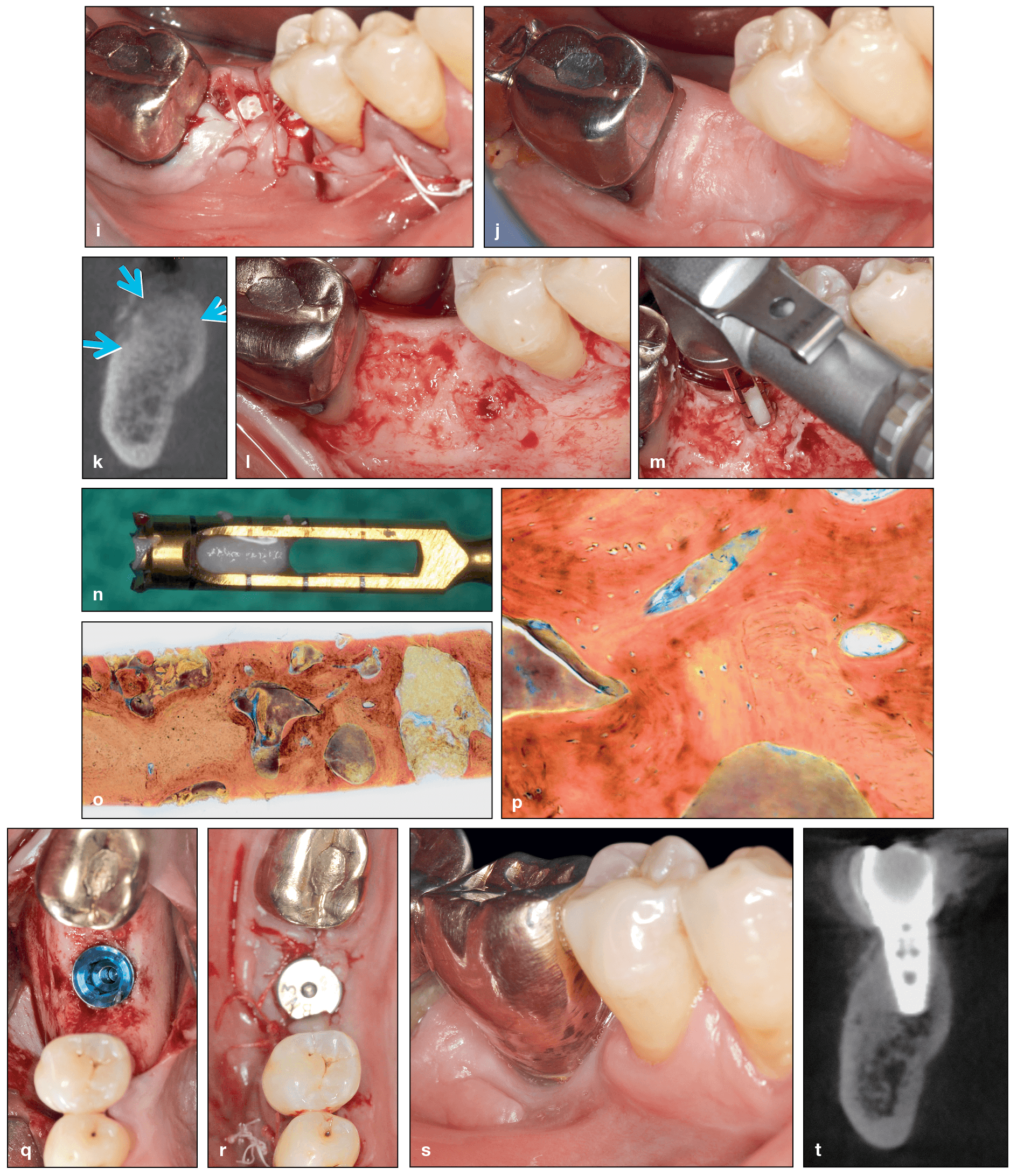
(cont) (i) Final sutures. (j and k) At 7 months postoperative, the ridge was maintained in both the horizontal and vertical dimensions. (l to n) A flap was raised, and a bone core sample was harvested. (o and p) Histology demonstrating excellent new bone formation with viable osteocytes following a 7-month healing period. (q and r) Implant placement. (s) Final restoration at 5 years. (t) CBCT at 5 years demonstrating excellent bone maintenance, especially on the buccal contour of the implant. (Case performed by Dr Michael A. Pikos.)
“I have also done a lot of research with rhBMP-9,” Dr Miron continues, “and that research is very fascinating. What happens with rhBMP-9 versus rhBMP-2, which is the current standard, is that you can use 5 to 20 times less rhBMP-9, and it’s more effective. The advantage then is that you’re giving your patients less of a medication or drug, which is always better, and it should theoretically cost less because you’re using less growth factor. One other area I find presents itself as having huge potential is all the work that has been done with Tetranite, a novel bone adhesive that is still in the preclinical testing phase. It’s basically a bone glue that is very biocompatible, and it allows you to place an implant and obtain primary stability where previously you may not have been able to place it. Tetranite adheres both to the implant metal as well as to bone and provides primary stability: ISQ values are excellent, they remain high over time, and it’s fully resorbable and replaced with native bone within a year, making it an absolutely dream product for dentistry if all the data holds up during clinical testing.”
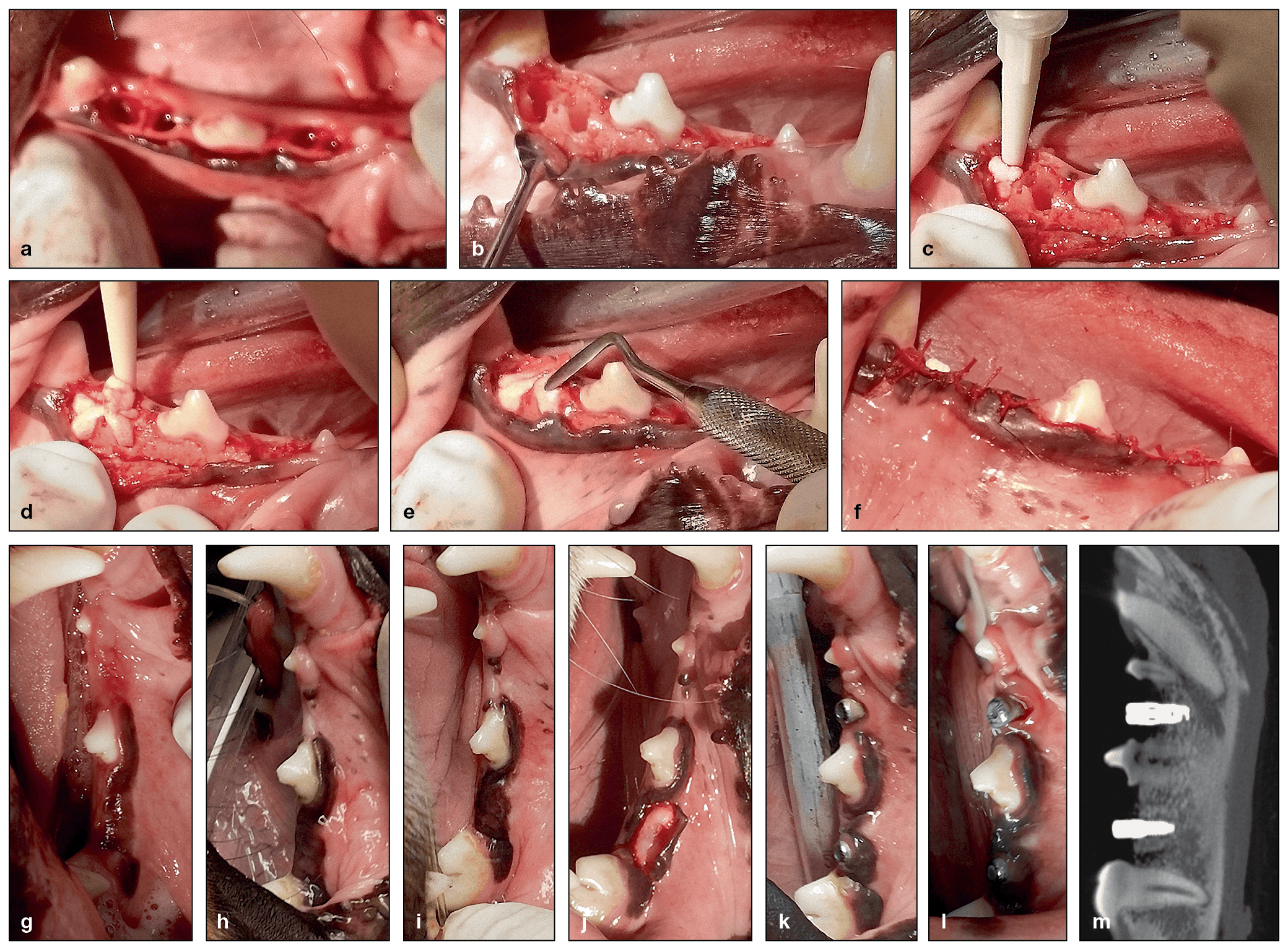
(a to f) Clinical photographs of a canine alveolar ridge that was preserved by injecting Tetranite into the extraction sockets. (g to i) Postoperative healing at 2 weeks (g), 6 weeks (h), and 9 weeks (i). Note the uneventful healing of the soft tissues and the overgrowth of the exposed material at week 2 (g). (j) Exposed ridge prior to osteotomy preparation at 12 weeks. (k and l) Clinical views 3 weeks and 9 weeks after implant placement, respectively. (m) CBCT image of the implants 26 weeks after injection of the Tetranite and 14 weeks after implant placement. The posterior implant is successfully osseointegrated, while the anterior site is too shallow for a successful outcome.
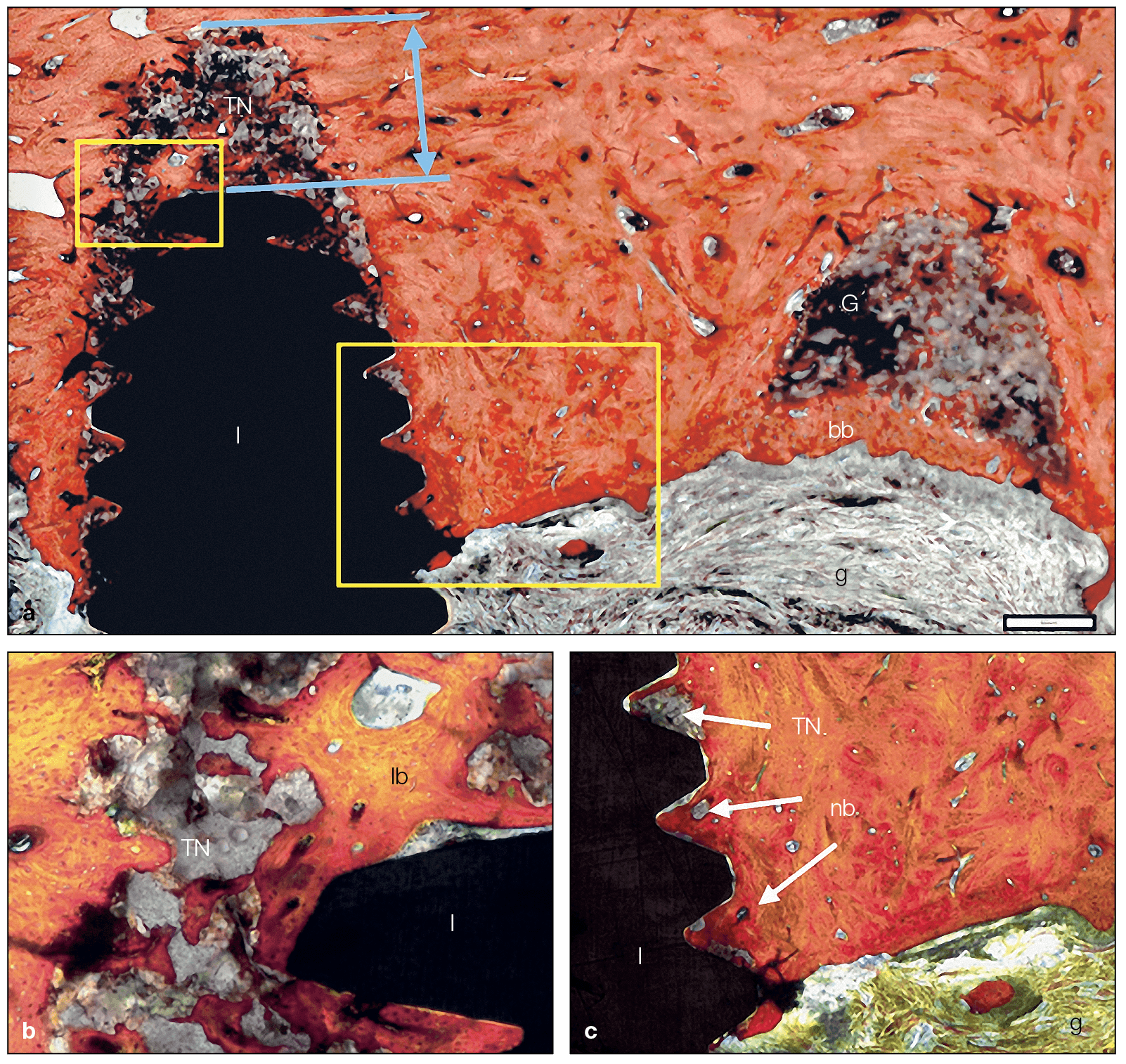
(a) Harvested tissue at week 26 demonstrates that TN integrates into host bone around implants even when β-TCP granules are added (ground section, trichrome stain). (b and c) Higher-magnification views of the areas indicated by the rectangles in a. After 26 weeks, TN is fully biocompatible and integrates into host bone. Furthermore, resorption of the material is observed with visual bone-to-implant contact. I, implant; nb, new bone; G, graft; bb, bony bridge; g, gingival tissue; lb, lamellar bone.
The Future Is Now: Moving Forward
The sheer number of all of these new and exciting biomaterials can no doubt feel overwhelming for the clinician who just wants to better treat their patients. But Dr Miron, who also teaches courses on these biomaterials, knows exactly how to guide these clinicians through the unfamiliar.
“In my experience, clinicians in general don’t just want to be told which material to use. They actually enjoy understanding how and why to use each material, why they’re used with different protocols, why you need BMPs here, and why you need a xenograft there. So with this book we really just wanted to answer the why, why, why. Why is Istvan Urban using autogenous bone with xenografts? Why is Daniel Buser doing contour augmentation? Why is Anton Sculean using hyaluronic acid? Why are Michael Pikos and Robert Marx using BMPs? There are real biologic reasons for using different biomaterials in different clinical indications. At the same time, we wanted to write it in a way that would feel more like a general story that you can read through with cases from a number of expert clinicians who have contributed, and the information just flows naturally. The science is there because Yufeng Zhang and I have extensively investigated each of these biomaterials, but the clinical practicality is also there throughout because we are also clinicians and, more importantly, because we collaborated on this project with colleagues whom I consider to be the top clinicians from around the world.”
No one can save every single tooth. But with better biomaterials and appropriate selection, we can surely get closer to achieving these idea results.
Similar to the sheer number of biomaterials introduced is the staggering amount of “information” about them, and that can be a problem for clinicians who are trying to understand the full picture about their biomaterial options.
Clinicians don’t want nonsense anymore.
Next-Generation Biomaterials for Bone & Periodontal Regeneration will no doubt open a new door into the future for clinicians worldwide. But just like no implant can guarantee success without the hands of a skilled surgeon, no biomaterial alone can guarantee successful bone or periodontal regeneration. A successful outcome will always rely on a skilled and knowledgeable clinician. What this book will do is increase the depth of the reader’s knowledge base and enable them to make more informed and evidence-based decisions about their biomaterials selection depending on the case, treatment, surgery, and their patient.
 Dr Richard J. Miron is currently Visiting Faculty in the Department of Periodontology at the University of Bern in Switzerland, where he completed his PhD studies. He has published over 150 peer-reviewed articles and lectures internationally on many topics relating to growth factors, bone biomaterials, and guided bone regeneration. Dr Miron has been awarded many top international prizes in regenerative dentistry and implant dentistry, including the ITI André Schroeder Research Prize, the International Association for Dental Research Young Investigator of the Year award in the field of implant dentistry, and the American Academy of Implant Dentistry Young Investigator grant award. He has completed postdoctoral research fellowships in Switzerland, Canada, China, Spain, and the United States.
Dr Richard J. Miron is currently Visiting Faculty in the Department of Periodontology at the University of Bern in Switzerland, where he completed his PhD studies. He has published over 150 peer-reviewed articles and lectures internationally on many topics relating to growth factors, bone biomaterials, and guided bone regeneration. Dr Miron has been awarded many top international prizes in regenerative dentistry and implant dentistry, including the ITI André Schroeder Research Prize, the International Association for Dental Research Young Investigator of the Year award in the field of implant dentistry, and the American Academy of Implant Dentistry Young Investigator grant award. He has completed postdoctoral research fellowships in Switzerland, Canada, China, Spain, and the United States.
 Dr Yufeng Zhang is the Luojiashan Distinguished Professor and chief physician at the Wuhan University Medical and Dental School in Wuhan, China. He completed a two-year peri-implant bone biology research fellowship at Queensland University of Technology in Brisbane, Australia, and in 2010 he completed an ITI scholar position at the University of Bern in Switzerland. His research is primarily engaged in the field of implanted biomaterials used for alveolar bone and periodontal regeneration. Dr Zhang was one of the first researchers to propose the concept of gene-activated tissue engineering scaffolds, developing a scaffold-gene integration system that has its own biologic activity in vivo due to the autocrine growth factor. He has since been responsible for the development of several biomaterials with numerous industrial partners.
Dr Yufeng Zhang is the Luojiashan Distinguished Professor and chief physician at the Wuhan University Medical and Dental School in Wuhan, China. He completed a two-year peri-implant bone biology research fellowship at Queensland University of Technology in Brisbane, Australia, and in 2010 he completed an ITI scholar position at the University of Bern in Switzerland. His research is primarily engaged in the field of implanted biomaterials used for alveolar bone and periodontal regeneration. Dr Zhang was one of the first researchers to propose the concept of gene-activated tissue engineering scaffolds, developing a scaffold-gene integration system that has its own biologic activity in vivo due to the autocrine growth factor. He has since been responsible for the development of several biomaterials with numerous industrial partners.
 Next-Generation Biomaterials for Bone & Periodontal Regeneration
Next-Generation Biomaterials for Bone & Periodontal Regeneration
Edited by Richard J. Miron and Yufeng Zhang
New and innovative biomaterials are being discovered or created in laboratories at an unprecedented rate, but many of them remain entirely foreign to practicing clinicians. This book addresses this gap in knowledge by summarizing some of the groundbreaking research performed to date on this topic and providing case examples of these biomaterials at work. The book begins with a review of the biologic background and applications of bone grafting materials utilized in dentistry. The principles of guided tissue and bone regeneration are covered in detail, including many recent advancements in barrier membrane technologies as well as use of platelet-rich fibrin and various growth factors, and many next-generation materials that will optimize future bone and periodontal regeneration are presented. The final chapter is designed to help clinicians select appropriate biomaterials for each specific regenerative protocol. Much like one implant size and shape cannot be utilized for every indication in implant dentistry, one bone grafting material, barrier membrane, or growth factor cannot maximize regenerative outcomes in all clinical situations. This textbook teaches clinicians how to utilize biomaterials in an appropriate, predictable, and evidence-based manner.
384 pp; 960 illus; ©2019; ISBN 978-0-86715-796-3 (B7963); $218
This article was written by Caitlin Davis, Quintessence Publishing.
©2019 BY QUINTESSENCE PUBLISHING CO, INC. PRINTING OF THIS DOCUMENT IS RESTRICTED TO PERSONAL USE ONLY. NO PART MAY BE REPRODUCED OR TRANSMITTED IN ANY FORM WITHOUT WRITTEN PERMISSION FROM THE PUBLISHER.

Pingback: Quintessence Roundup: February | Quintessence Publishing Blog
Pingback: Quintessence Roundup: March | Quintessence Publishing Blog
Pingback: Quintessence Roundup: April | Quintessence Publishing Blog
Pingback: Quintessence Roundup: May | Quintessence Publishing Blog